Water Chemistry.
- kkorotev
- Posts: 163
- Joined: 23 May 2004, 00:07
- I've donated: $50.00!
- My articles: 1
- My images: 18
- Spotted: 11
- Location 1: Milwaukee, Wisconsin USA
Water Chemistry.
Theoretically, if r/o water has had everything removed, it should have neutral pH?
Any deviation from 7 would mean there's something else in there creating acidic or alkaline measurements...OR your method for measuring is incorrect?
Thoughts?
Any deviation from 7 would mean there's something else in there creating acidic or alkaline measurements...OR your method for measuring is incorrect?
Thoughts?
- racoll
- Posts: 5258
- Joined: 26 Jan 2004, 12:18
- My articles: 6
- My images: 182
- My catfish: 2
- My cats species list: 2 (i:2, k:0)
- My aquaria list: 1 (i:0)
- Spotted: 238
- Location 1: London
- Location 2: UK
Re: Water Chemistry.
Yes, in theory it should be 7. However, with no alkalinity, even the ambient CO2 dissolved from the air will cause a pH drop. Also, hobbyist RO units do not remove absolutely everything, like in analytical grade water, so some ions may remain which also reduce pH slightly.
- kkorotev
- Posts: 163
- Joined: 23 May 2004, 00:07
- I've donated: $50.00!
- My articles: 1
- My images: 18
- Spotted: 11
- Location 1: Milwaukee, Wisconsin USA
Re: Water Chemistry.
SO...
If my (straight from the new r/o unit [flushed for 4 hours] is reading +8 pH, my Hannah digital reader should be re-calibrated?
...and I should use my new r/o water as the calibration solution for "neutral-or near enough" pH?
Thanks, racoll...very much, for your response.
Kevin
If my (straight from the new r/o unit [flushed for 4 hours] is reading +8 pH, my Hannah digital reader should be re-calibrated?
...and I should use my new r/o water as the calibration solution for "neutral-or near enough" pH?
Thanks, racoll...very much, for your response.
Kevin
- racoll
- Posts: 5258
- Joined: 26 Jan 2004, 12:18
- My articles: 6
- My images: 182
- My catfish: 2
- My cats species list: 2 (i:2, k:0)
- My aquaria list: 1 (i:0)
- Spotted: 238
- Location 1: London
- Location 2: UK
Re: Water Chemistry.
No, I remember exactly the same thing happened to me. I think it is the membrane preservative fluid that make the pH go high. It goes away after a while, but can't remember how long exactly.If my (straight from the new r/o unit [flushed for 4 hours] is reading +8 pH, my Hannah digital reader should be re-calibrated?
No, you should use the proprietary calibration fluids, and follow the instructions exactly.and I should use my new r/o water as the calibration solution for "neutral-or near enough" pH?
-
Bas Pels
- Posts: 2917
- Joined: 21 Dec 2006, 20:35
- My images: 1
- My cats species list: 28 (i:0, k:0)
- Spotted: 8
- Location 1: the Netherlands
- Location 2: Nijmegen the Netherlands
- Interests: Central American and Uruguayan fishes
Re: Water Chemistry.
As the RO unit prevents gasses from passing, the water - straight from the unit - should not have the normal pH value for demineralized water, approx 5 due to dissolved carbon oxide. Because this gas has been remouved.
If you distrust the pH meter, take a sample of the water and a straw. Gentle blow through the water and the CO2 in your preath will push the pH down, easily to a value of 4.
You should never use this RO water directly as it is also free of oxygen. A few hours with a blowing stone will help this. Mixing it with tap water will suffice as wel
If you distrust the pH meter, take a sample of the water and a straw. Gentle blow through the water and the CO2 in your preath will push the pH down, easily to a value of 4.
You should never use this RO water directly as it is also free of oxygen. A few hours with a blowing stone will help this. Mixing it with tap water will suffice as wel
cats have whiskers
-
dw1305
- Posts: 1104
- Joined: 22 Oct 2009, 11:57
- Location 1: Corsham, UK
- Location 2: Bath, UK
- Interests: Natural History, Ecology, Plants, Biotopes, Taxonomy, Nitrification, Cricket & Northern Soul
Re: Water Chemistry.
Hi all,
pH is probably both the most misunderstood and problematic measurement we use. This is really important, you can't calibrate the meter, or get it a meaningful pH reading from RO water.
In fact I would go further than this pH is a meaningless reading unless you have some measure of the carbonate buffering (dKH) of the water. One of the great difference between keeping black water fish and Rift lake fish is that in water which is close to being pure H2O, pH is a totally meaningless measurement. This is a difficult concept if you are used to working with infinitely carbonate buffered water, where relatively small changes in pH are potentially damaging to the fish, and are caused by huge changes in the balance of acid and alkaline ions.
The pH meter
What people often don't realise is that you can't just dip a pH meter into a tank and get an accurate reading, they are quite complex bits of kit. They need to be left to equilibrate before reading, and if you have low conductivity water this may take a long time. If you have low conductivity water combined with little carbonate buffering, the pH will be inherently unstable and will fluctuate wildly with every addition, however small, of acid or base (This is the CO2 in RO water scenario). pH only tells us the ratio of acid and bases, not the quantity.
Buffer calibration solutions.
These are the 2 x buffers for pH4 and pH7 (possibly coloured red and yellow). These are what they say, "buffers to changes in pH", they are often an mix of 0.2M Na2HPO4 & 0.1M Citric Acid, by varying the proportion of these in the buffer you can cover pH3 to pH8. You need to replace them if you think they have been diluted with water, but within reason they should always read "pH4" and "pH7". You need these to calibrate your pH meter.
In the tank there are processes that buffer water by adding or removing H+ ions. The pH is the ratio of the different weights and tells you nothing about the amounts.
This is the "alkaline water" model
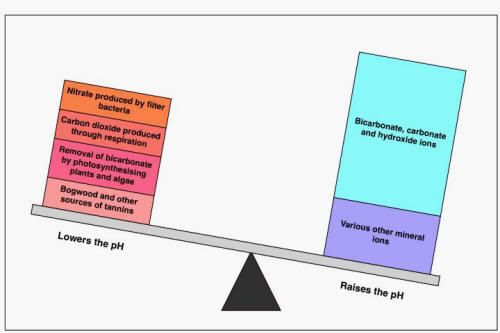
and this id the "acidic water" model
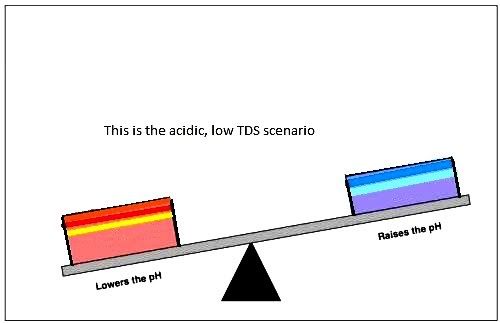
pH range
pH will always be more prone to vary between pH6 and PH8, as we are only talking change from 10-6 to 10-8 in the H+ ion (and 10-8 to 10-6 in OH- ion) concentration. But if we go from pH6 to pH5 we have gone from 10-6 to 10-5 H+ (and 10-8 to 10-9 OH- ion conc.)

pH the measure
Water is subject to a self-ionization process ~ H2O is in equilibrium with H+ + OH−. pH is a bit of a strange measurement, it is a measure of the ration of H+ ions to OH- ions expressed as the "negative decimal logarithm of the hydrogen ion activity in a solution" in the case of pH7 it is 0.0000001M H+
(1 x 10-7 H+ ions) and also 0.0000001M OH- ions. This range of pH0 - pH14 is the possible H+ ions from 1.0M (pH0) through to 0.0000000000000001M (pH14). The dissociation constant, KW, has a value of about 10−14, so, in neutral solution of a salt, both the hydrogen ion concentration and hydroxide ion concentration are about 10−7 Mol l-1.
Reference electrode.
The reference electrode membrane is the most likely point of failure. These are usually combined glass and "Calomel" reference electrodes "is a reference electrode based on the reaction between elemental mercury and mercury(I) chloride. The aqueous phase in contact with the mercury and the mercury(I) chloride (Hg2Cl2, "calomel") is a saturated solution of potassium chloride (KCl) in water <http://en.wikipedia.org/wiki/Saturated_ ... _electrode>." This is where the KCl comes in, you need to store the electrode in dilute KCl, and if it dries out, you may be able to resuscitate it with a stronger KCl solution. Storing the probe in the buffer or RO may have damaged the electrode.
cheers Darrel
If my (straight from the new r/o unit [flushed for 4 hours] is reading +8 pH, my Hannah digital reader should be re-calibrated?
...and I should use my new r/o water as the calibration solution for "neutral-or near enough" pH?
pH is probably both the most misunderstood and problematic measurement we use. This is really important, you can't calibrate the meter, or get it a meaningful pH reading from RO water.
In fact I would go further than this pH is a meaningless reading unless you have some measure of the carbonate buffering (dKH) of the water. One of the great difference between keeping black water fish and Rift lake fish is that in water which is close to being pure H2O, pH is a totally meaningless measurement. This is a difficult concept if you are used to working with infinitely carbonate buffered water, where relatively small changes in pH are potentially damaging to the fish, and are caused by huge changes in the balance of acid and alkaline ions.
The pH meter
What people often don't realise is that you can't just dip a pH meter into a tank and get an accurate reading, they are quite complex bits of kit. They need to be left to equilibrate before reading, and if you have low conductivity water this may take a long time. If you have low conductivity water combined with little carbonate buffering, the pH will be inherently unstable and will fluctuate wildly with every addition, however small, of acid or base (This is the CO2 in RO water scenario). pH only tells us the ratio of acid and bases, not the quantity.
Buffer calibration solutions.
These are the 2 x buffers for pH4 and pH7 (possibly coloured red and yellow). These are what they say, "buffers to changes in pH", they are often an mix of 0.2M Na2HPO4 & 0.1M Citric Acid, by varying the proportion of these in the buffer you can cover pH3 to pH8. You need to replace them if you think they have been diluted with water, but within reason they should always read "pH4" and "pH7". You need these to calibrate your pH meter.
In the tank there are processes that buffer water by adding or removing H+ ions. The pH is the ratio of the different weights and tells you nothing about the amounts.
This is the "alkaline water" model

and this id the "acidic water" model

pH range
pH will always be more prone to vary between pH6 and PH8, as we are only talking change from 10-6 to 10-8 in the H+ ion (and 10-8 to 10-6 in OH- ion) concentration. But if we go from pH6 to pH5 we have gone from 10-6 to 10-5 H+ (and 10-8 to 10-9 OH- ion conc.)

pH the measure
Water is subject to a self-ionization process ~ H2O is in equilibrium with H+ + OH−. pH is a bit of a strange measurement, it is a measure of the ration of H+ ions to OH- ions expressed as the "negative decimal logarithm of the hydrogen ion activity in a solution" in the case of pH7 it is 0.0000001M H+
(1 x 10-7 H+ ions) and also 0.0000001M OH- ions. This range of pH0 - pH14 is the possible H+ ions from 1.0M (pH0) through to 0.0000000000000001M (pH14). The dissociation constant, KW, has a value of about 10−14, so, in neutral solution of a salt, both the hydrogen ion concentration and hydroxide ion concentration are about 10−7 Mol l-1.
Reference electrode.
The reference electrode membrane is the most likely point of failure. These are usually combined glass and "Calomel" reference electrodes "is a reference electrode based on the reaction between elemental mercury and mercury(I) chloride. The aqueous phase in contact with the mercury and the mercury(I) chloride (Hg2Cl2, "calomel") is a saturated solution of potassium chloride (KCl) in water <http://en.wikipedia.org/wiki/Saturated_ ... _electrode>." This is where the KCl comes in, you need to store the electrode in dilute KCl, and if it dries out, you may be able to resuscitate it with a stronger KCl solution. Storing the probe in the buffer or RO may have damaged the electrode.
cheers Darrel
- MatsP
- Posts: 21038
- Joined: 06 Oct 2004, 13:58
- My articles: 4
- My images: 28
- My cats species list: 117 (i:33, k:0)
- My aquaria list: 10 (i:8)
- My BLogs: 4 (i:0, p:164)
- Spotted: 187
- Location 1: North of Cambridge
- Location 2: England.
Re: Water Chemistry.
Assuming the RO unit is like most common ones, where water comes out at a rate of "just enough to not form individual drops", I'd think that the water gets reasonably oxygenated from running down into the storage container [of course, in a bizarre world, you could somehow have arranged for a nitrogen gas cylinder to keep the storage container oxygen free - but that doesn't seem very plausible].Bas Pels wrote:You should never use this RO water directly as it is also free of oxygen. A few hours with a blowing stone will help this. Mixing it with tap water will suffice as wel
Obviously, if the RO unit is producing several thousand liters a day, and you just pour water straight from the RO unit directly into the tank, I'd say there may be something to id. But if it's been slowly filling a container for many hours, not a problem.
Also, you'd probably mix up the RO water with air (and thus oxygen) when it's poured into the tank.
I have been filling my tank directly from the loft-tank where it's stored since I got my RO unit about 15 months ago, and not had any noticeable problems with "low oxygen".
--
Mats
- MatsP
- Posts: 21038
- Joined: 06 Oct 2004, 13:58
- My articles: 4
- My images: 28
- My cats species list: 117 (i:33, k:0)
- My aquaria list: 10 (i:8)
- My BLogs: 4 (i:0, p:164)
- Spotted: 187
- Location 1: North of Cambridge
- Location 2: England.
Re: Water Chemistry.
It's also possible that the small amount of mineral content that has passed through the unit is enough to raise the pH a little bit. As Darrel explains below, there is very little buffering capacity in the RO water, so it only needs a SMALL amount of something else to make the water acidic _OR_ alkaline.racoll wrote:No, I remember exactly the same thing happened to me. I think it is the membrane preservative fluid that make the pH go high. It goes away after a while, but can't remember how long exactly.If my (straight from the new r/o unit [flushed for 4 hours] is reading +8 pH, my Hannah digital reader should be re-calibrated?
No, you should use the proprietary calibration fluids, and follow the instructions exactly.and I should use my new r/o water as the calibration solution for "neutral-or near enough" pH?
--
Mats
- Shane
- Expert
- Posts: 4646
- Joined: 30 Dec 2002, 22:12
- My articles: 69
- My images: 162
- My catfish: 75
- My cats species list: 4 (i:0, k:0)
- My aquaria list: 4 (i:4)
- Spotted: 99
- Location 1: Tysons
- Location 2: Virginia
- Contact:
Re: Water Chemistry.
Darrel,
Great post. Many thanks for taking the time to put that together.
-Shane
Great post. Many thanks for taking the time to put that together.
-Shane
"My journey is at an end and the tale is told. The reader who has followed so faithfully and so far, they have the right to ask, what do I bring back? It can be summed up in three words. Concentrate upon Uganda."
Winston Churchill, My African Journey
Winston Churchill, My African Journey
-
Viktor Jarikov
- Posts: 5583
- Joined: 26 Jan 2010, 20:11
- My images: 11
- My cats species list: 25 (i:0, k:0)
- Spotted: 4
- Location 1: Naples, FL
- Location 2: USA
Re: Water Chemistry.
I think the term "activity" here can be simply replaced by "molarity" as the valence of the hydrogen cation is one. Activity = molarity x valence. If I am correct, this would exclude the term activity which is not touched upon in this nice layout.dw1305 wrote:pH is a bit of a strange measurement, it is a measure of the ration of H+ ions to OH- ions expressed as the "negative decimal logarithm of the hydrogen ion activity in a solution" in the case of pH7 it is 0.0000001M H+ (1 x 10-7 H+ ions) and also 0.0000001M OH- ions.
Thebiggerthebetter
fish-story.com
fish-story.com
- Jools
- Expert
- Posts: 16268
- Joined: 30 Dec 2002, 15:25
- My articles: 198
- My images: 941
- My catfish: 237
- My cats species list: 87 (i:235, k:1)
- My BLogs: 7 (i:10, p:167)
- My Wishlist: 23
- Spotted: 450
- Location 1: Middle Earth,
- Location 2: Scotland
- Interests: All things aquatic, Sci-Fi, photography and travel. Oh, and beer.
- Contact:
Re: Water Chemistry.
I agree, it's one of the best I've read in ages.Shane wrote:Darrel,
Great post. Many thanks for taking the time to put that together.
-Shane
Cheers,
Acid Jools
Owner, AquaticRepublic.com, PlanetCatfish.com & ZebraPleco.com. Please consider donating towards this site's running costs.
- racoll
- Posts: 5258
- Joined: 26 Jan 2004, 12:18
- My articles: 6
- My images: 182
- My catfish: 2
- My cats species list: 2 (i:2, k:0)
- My aquaria list: 1 (i:0)
- Spotted: 238
- Location 1: London
- Location 2: UK
Re: Water Chemistry.
Yes, absolutely.Jools wrote: I agree, it's one of the best I've read in ages.
Darrel, what are your thoughts about using straight RO in an aquarium for blackwater species? You hear time and time again that you shouldn't do it because the pH can "crash", but I'm fairly sure that's nonsense. I've been running tanks on neat RO for ages, and had no problems whatsoever.
-
dw1305
- Posts: 1104
- Joined: 22 Oct 2009, 11:57
- Location 1: Corsham, UK
- Location 2: Bath, UK
- Interests: Natural History, Ecology, Plants, Biotopes, Taxonomy, Nitrification, Cricket & Northern Soul
Re: Water Chemistry.
Hi all,
Thanks for the kind comments, I know they aren't the best explanations of pH and buffering, but I've really struggled to find words that covers the important bits without becoming too technical, this is both on forums and in my day job.
I also don't think there is a problem with using pure RO, as long as you have some humic compounds in the water (from dead leaves, peat filtration etc.), and have fairly low stocking to take into account the low levels of microbial activity in nutrient poor, acid conditions. I've done this, but I only keep planted tanks, and plants make water management much, much easier. Despite what is often written, there are quite a wide range of plants that will grow in very nutrient poor acid conditions, as well as extreme calcifuges like Tonina and Isoetes, most "low light" plants (Anubias, mosses, ferns, Cryptocoryne spp. etc) will grow slowly without any problem.
I use a lot of Ceratopteris in very soft water, it is ideal as fry habitat, has access to aerial CO2 etc. Utricularia gibba is another one I like, although it isn't to every ones taste.
cheers Darrel
Thanks for the kind comments, I know they aren't the best explanations of pH and buffering, but I've really struggled to find words that covers the important bits without becoming too technical, this is both on forums and in my day job.
Yes it can, activity is the correct term, but Viktor is correct that activity = molarity.I think the term "activity" here can be simply replaced by "molarity" as the valence of the hydrogen cation is one. Activity = molarity x valence.
I'm pretty sure this is true as well, it will depend upon surface area to volume ratio of the storage vessel etc, but I would expect that the water will be fully oxygenated.Assuming the RO unit is like most common ones, where water comes out at a rate of "just enough to not form individual drops", I'd think that the water gets reasonably oxygenated from running down into the storage container
I've had a go at the "pH crash" scenario a couple of times before. If you read the threads, quite a few respected plec breeders didn't agree, but personally I think you can safely totally ignore the "pH crash" scenario. "My 183's mated and I didnt even know it!!!" <http://www.planetcatfish.com/forum/view ... h&start=20> and "T.D.S ?"<http://www.planetcatfish.com/forum/view ... =5&t=32162>.Darrel, what are your thoughts about using straight RO in an aquarium for blackwater species? You hear time and time again that you shouldn't do it because the pH can "crash", but I'm fairly sure that's nonsense. I've been running tanks on neat RO for ages, and had no problems whatsoever.
I also don't think there is a problem with using pure RO, as long as you have some humic compounds in the water (from dead leaves, peat filtration etc.), and have fairly low stocking to take into account the low levels of microbial activity in nutrient poor, acid conditions. I've done this, but I only keep planted tanks, and plants make water management much, much easier. Despite what is often written, there are quite a wide range of plants that will grow in very nutrient poor acid conditions, as well as extreme calcifuges like Tonina and Isoetes, most "low light" plants (Anubias, mosses, ferns, Cryptocoryne spp. etc) will grow slowly without any problem.
I use a lot of Ceratopteris in very soft water, it is ideal as fry habitat, has access to aerial CO2 etc. Utricularia gibba is another one I like, although it isn't to every ones taste.
cheers Darrel




