Hi all,
If my (straight from the new r/o unit [flushed for 4 hours] is reading +8 pH, my Hannah digital reader should be re-calibrated?
...and I should use my new r/o water as the calibration solution for "neutral-or near enough" pH?
pH is probably both the most misunderstood and problematic measurement we use. This is really important,
you can't calibrate the meter, or get it a meaningful pH reading from RO water.
In fact I would go further than this
pH is a meaningless reading unless you have some measure of the carbonate buffering (dKH) of the water. One of the great difference between keeping black water fish and Rift lake fish is that in water which is close to being pure H2O, pH is a totally meaningless measurement. This is a difficult concept if you are used to working with infinitely carbonate buffered water, where relatively small changes in pH are potentially damaging to the fish, and are caused by huge changes in the balance of acid and alkaline ions.
The pH meter
What people often don't realise is that you can't just dip a pH meter into a tank and get an accurate reading, they are quite complex bits of kit. They need to be left to equilibrate before reading, and if you have low conductivity water this may take a long time. If you have low conductivity water combined with little carbonate buffering, the pH will be inherently unstable and will fluctuate wildly with every addition, however small, of acid or base (This is the CO2 in RO water scenario).
pH only tells us the ratio of acid and bases, not the quantity.
Buffer calibration solutions.
These are the 2 x buffers for pH4 and pH7 (possibly coloured red and yellow). These are what they say, "buffers to changes in pH", they are often an mix of 0.2M Na2HPO4 & 0.1M Citric Acid, by varying the proportion of these in the buffer you can cover pH3 to pH8. You need to replace them if you think they have been diluted with water, but within reason they should always read "pH4" and "pH7".
You need these to calibrate your pH meter.
In the tank there are processes that buffer water by adding or removing H+ ions. The pH is the ratio of the different weights and tells you nothing about the amounts.
This is the "alkaline water" model
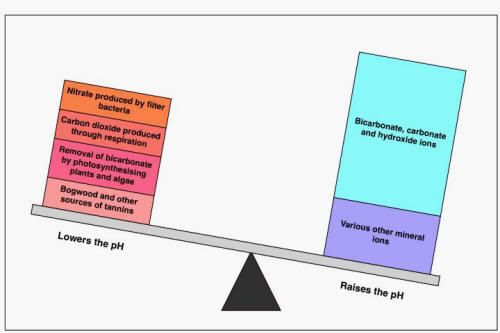
and this id the "acidic water" model
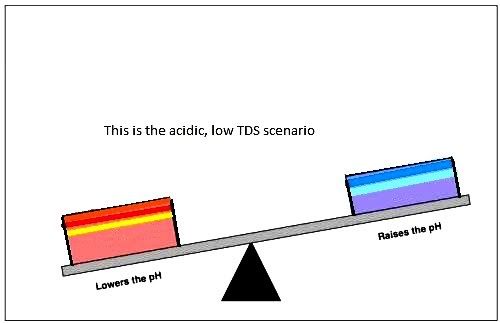 pH range
pH range
pH will always be more prone to vary between pH6 and PH8, as we are only talking change from 10-6 to 10-8 in the H+ ion (and 10-8 to 10-6 in OH- ion) concentration. But if we go from pH6 to pH5 we have gone from 10-6 to 10-5 H+ (and 10-8 to 10-9 OH- ion conc.)
 pH the measure
pH the measure
Water is subject to a self-ionization process ~ H2O is in equilibrium with H+ + OH−. pH is a bit of a strange measurement, it is a measure of the ration of H+ ions to OH- ions expressed as the "negative decimal logarithm of the hydrogen ion activity in a solution" in the case of pH7 it is 0.0000001M H+
(1 x 10-7 H+ ions) and also 0.0000001M OH- ions. This range of pH0 - pH14 is the possible H+ ions from 1.0M (pH0) through to 0.0000000000000001M (pH14). The dissociation constant, KW, has a value of about 10−14, so, in neutral solution of a salt, both the hydrogen ion concentration and hydroxide ion concentration are about 10−7 Mol l-1.
Reference electrode.
The reference electrode membrane is the most likely point of failure. These are usually combined glass and "Calomel" reference electrodes "is a reference electrode based on the reaction between elemental mercury and mercury(I) chloride. The aqueous phase in contact with the mercury and the mercury(I) chloride (Hg2Cl2, "calomel") is a saturated solution of potassium chloride (KCl) in water <
http://en.wikipedia.org/wiki/Saturated_ ... _electrode>." This is where the KCl comes in, you need to store the electrode in dilute KCl, and if it dries out, you may be able to resuscitate it with a stronger KCl solution. Storing the probe in the buffer or RO may have damaged the electrode.
cheers Darrel


